By Exploristics Chief Data Scientific Officer Kimberley Hacquoil
We live in an interconnected society where nothing in life exists in complete isolation. Everywhere we look there are things interacting with other things. For example, how are you feeling as you read this article? That may be as a result of all manner of things, how well you slept last night, what you ate for breakfast, how much social interaction you have had today etc. It is no different within drug discovery and development. It is a multifaceted process of identifying new drugs with the aim to provide patients with safe and efficacious medicines. It’s a tough, long, and complex process which has many potential routes to work through. This is one of the reasons why I find this industry fascinating and constantly challenging thus rewarding.
Interactions to identify connections
In life, correlations express the mutual relationship or connection between two things. Similarly, as a statistical term it’s defined as a connection or relationship between two or more facts, numbers.
Working in multi-disciplinary project teams, I have often had many stimulating conversations with colleagues regarding different factors which may impact development plans. We have spent time diligently identifying and considering a wide range of elements from biology features to clinical aspects, to commercialisation implications. Project teams will raise key questions which highlights what relationships or correlations are needed to be understood to address them. Questions like:
| Question
|
Correlation |
| How will the biology translate to the clinical setting? | Correlation between biological and clinical outcomes |
| Which patient population are most likely to benefit from this experimental medicine? i.e., inform inclusion/exclusion | Correlation between risk factors and outcome of interest |
| Can we get information from an earlier timepoint/endpoint to support decision-making? | Correlation between different outcomes and/or time-points |
| What baseline factors are predictive of treatment response in patients? | Correlation between baseline covariates and outcome of interest |
| What is the right dose level (both from an efficacy and safety standpoint)? | Correlation between dose and outcome |
| How can I best design a study to incorporate multiple decision criteria? e.g., regulatory requirements and payor requirements | Correlation between primary and secondary objectives/endpoints |
The answers to some of these questions are very drug specific but also often depend on other factors such as the strategic focus of a company or other broader considerations. But one thing they all have in common is the aim to take into consideration multiple factors which are thought to impact the success of a development program. It’s vital to understand how these factors interplay with each other and determine the best way to navigate through the uncertainties.
Current tactics to deal with interrelationships in clinical trials
In one sense, as an industry we account for certain correlations and interrelationships very well. For example, through randomisation and through different analysis approaches.
Randomisation is a technique to mitigate selection bias in clinical trials involving treatment comparisons. If correlations exist between baseline covariates and the outcome of interest, randomisation ensures that, on average, the baseline covariates are balanced between arms and thus observed differences between the arms can be attributed to the treatment.
Regulators have specific and comprehensive guidance for statisticians on how to adjust analyses for different covariates and factors which are anticipated to have an impact on the primary analysis. There has been a lot of technical research into this area and there are numerous different statistical methods which could be utilised here. Therefore, study teams will stipulate detailed plans within the analysis sections and assess different methods and approaches to minimise bias related to this. There is an appreciation that adjustment for relevant covariates and factors will increase precision and mitigate risks between any unbalance between different treatment groups. The important part here is that any covariates or factors are relevant – there is no benefit in just adjusting for multiple things in the analysis “just in case”, and in fact including too much can sometimes do harm. This comes back to the fact that it’s vital for project and study teams to identify these interrelationships to ensure this can be used appropriately in the design and analysis of a trial.
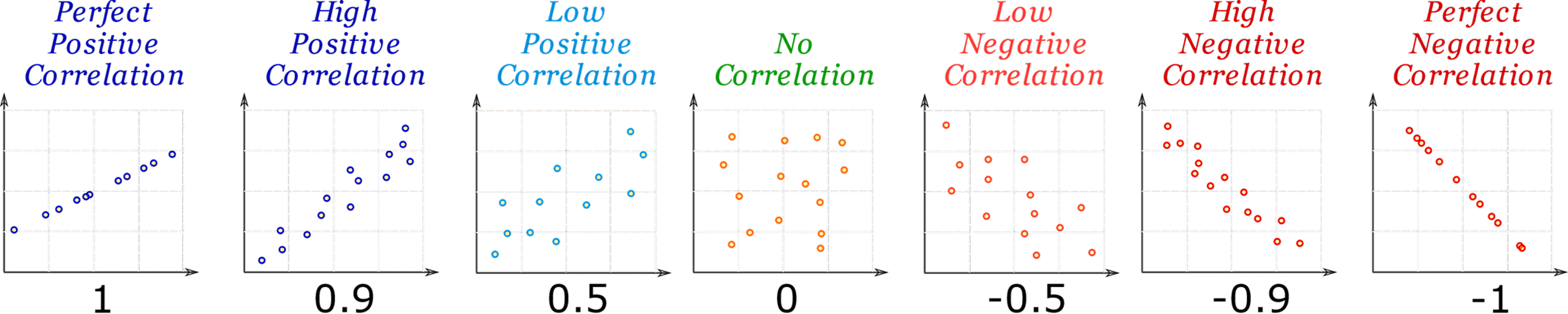
Figure: Visual representation of different correlations between two measures
A better understanding of inter-relationships to improve trial design
So much attention is rightly focussed on accounting for interrelationships when planning the analysis of the clinical trial data. However, we at Exploristics believe that the industry can improve by exploiting our knowledge about interrelationships at the trial design stage in a way we do not currently routinely do.
One example is with sample size calculations. As mentioned there has been a lot of focus on what is the best method for adjusting for covariates and factors of clinical trial data. However, at the design stage, this is often ignored when a simplified approach is used to determine the sample size. The simplification is to usually ignore the adjustment which will be used in the actual analysis. However, the regulators highlight the fact that adjusting the analysis for covariates and factors will improve precision. This means that we may very well have more statistical power than we think. This sounds great, doesn’t it? Although is this really an efficient and optimal approach to study design? We may be including more patients than we need, not choosing the best design or estimands strategy – all because we are not really designing the same trial we will in fact carry out and analyse. We can do much better to ensure less patients and more statistical power in the studies we design.
In truth, there are many other factors that are affected by correlations, which are usually ignored or over-simplified at the trial design stage. Another example is in confirmatory trials, where typically a sponsor will be hoping to demonstrate efficacy not only on the primary endpoint, but on as many key secondary endpoints as possible, too. These key secondary endpoints are likely to be correlated with each other and with the primary endpoint. If the fixed sequence method is used to test for efficacy in the key secondary endpoints, the order in which you perform the analyses is crucial to how many endpoints you may be able to declare efficacy on. Harnessing our knowledge of correlations at the design stage may impact the order in which we choose to analyse these endpoints. This could increase the probability of adding to the labelling claims.
Understanding the multiple correlations that might affect the outcome of a study is complex. It requires early statistical input into the design process. Bringing statisticians in earlier will help, as well as adopting study simulation techniques that can unpick the complicated relationships between different study factors. Using patient level simulation of clinical trials at the design stage ensures that the actual analyses which are going to be carried out on the real data can be done in silico. Hence all the research and effort that’s gone into the statistical techniques for the analysis can also be used for design as well. This will mean that correlations can be utilised appropriately to accurately quantify the likelihood of success of a trial.
Simulation will also enable teams to quantify any risks associated with different design options where interrelationships might be anticipated but not known for sure. It allows teams to determine which factors might have a greater impact on the probability of success and then mitigation plans or changes to the design can be appropriately built into the trial.
Therefore, simulation offers a valuable resource for project teams to inform study design with important information on key relationships between study factors. It can take their understanding to the next level by enabling them to quantify the impact of these relationships and so inform robust design decision-making. Going forward, quantifying the impact of interactions and inter-relationships more closely during the study design process will help project teams deliver better studies, and so find a more efficient way to develop the best medicines.
Read more:
From cars to clinical trials, how simulation can improve design
Embedding new technologies into clinical studies
Shifting the development paradigm with innovative trial design
Revolutionising clinical trial design with in silico studies using synthetic data
Statistical Consulting Services
Hear more:
The power of simulations for designing clinical studies and beyond
Simulations your most powerful study design tool
Watch more:
Why do correlations matter in clinical trial design?




