Case Study
Enabling early approval with a smarter approach to generating robust clinical evidence
KerusCloud is a revolutionary simulation-guided study design tool that ensures clinical trials are designed effectively to collect the right data, in the right patients, in the right way. Its use supports evidence-based design decisions to extensively de-risk real clinical studies, reducing development time, costs and patient burden.
The Challenge
A small biotechnology company with limited resources was developing a new antibacterial treatment for C. difficile (CDI). CDI is the most common single organism causing healthcare associated infections. In vulnerable patients, CDI infections have high mortality rates, ~30% for severe CDI and ~40% in elderly patients. The Sponsor was seeking early access for patients via the breakthrough therapy initiative in the US and medicines adaptive pathways (MAPPs) in the EU.
- A previous study assessment indicated that the development programme for the new antibacterial agent would need ~1000 patients. However, this development plan was impractical and could not be executed.
- How could evidence be generated to support rapid marketing authorisation?
The Approach
KerusCloud was used to evaluate alternative development plan options that would be feasible given the Sponsor’s constraints. To do this:
- Information was collated from a variety of sources, including data on multiple correlated endpoints comprising clinical and pharmacodynamic measurements.
- The data sourced was processed and then converted into synthetic data sets within KerusCloud to build virtual patient populations (Figure 1) that could inform study simulations.
- KerusCloud simulated thousands of studies with outcomes explored using an interactive heatmap (Figure 2) so that the impact of different study design parameters and what if scenarios could be assessed rapidly in silico.
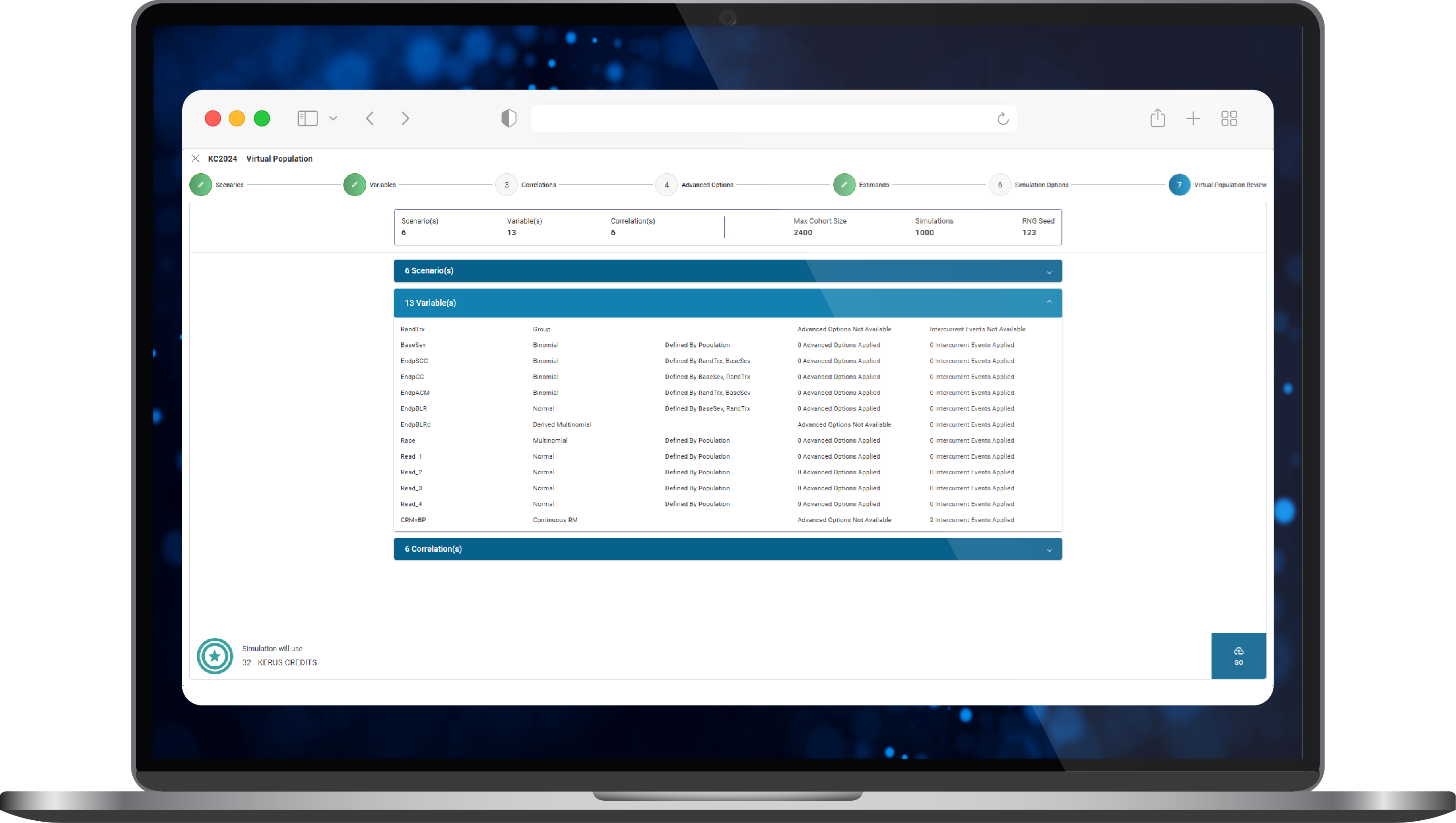
Figure 1. Construction of a virtual population in KerusCloud
The Results
- An alternative development plan was identified with the best design and endpoints for generating an evidence package for rapid approval.
- This showed that an initial evidence package could be generated using 180 rather than 1,000 patients.
- Design options and simulated evidence were presented to the FDA and EMA, so Regulators could give scientific advice on how best to proceed.
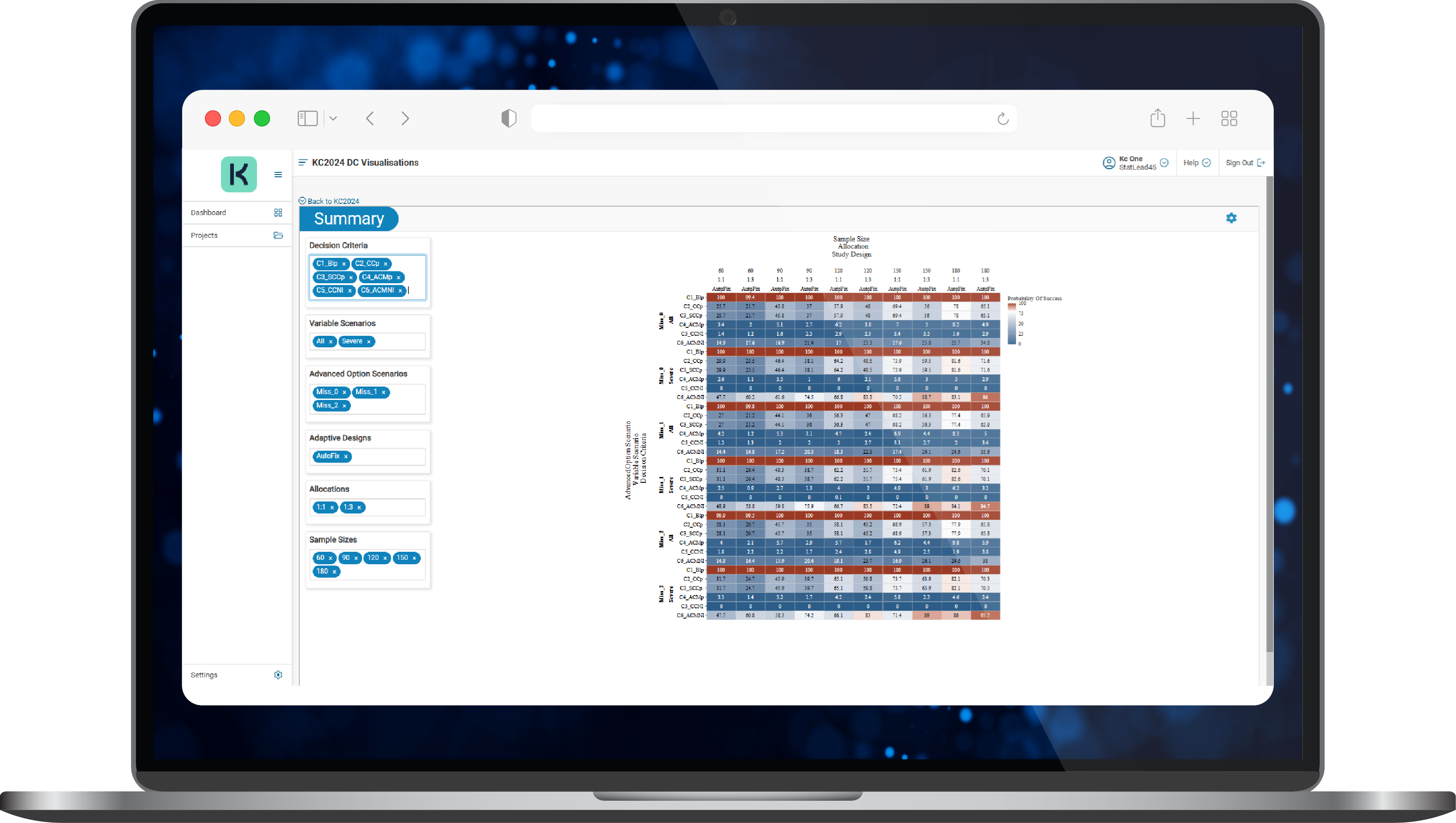
Figure 2. A typical results heatmap in KerusCloud
The Impact
KerusCloud provided an alternative development plan to accelerate the delivery of a new antibacterial treatment option to patients that:
- Provided evidence that Regulators agreed would likely be sufficient for approval.
- Reduced the time to market by 3-5 years.
- Saved the sponsor £18M in development costs.
“The KerusCloud simulation tool is very powerful! The simulations for our pivotal trials showed us a suitable and straight forward path to reach marketing approval with a smaller number of patients and quicker compared to our original plans. Discussions with statistic experts from CROs, investigators and key opinion leaders confirmed the approach.”
CEO, Small Biotech, Germany
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




