Case Study
Establishing biomarkers of treatment efficacy in liver disease
KerusCloud is a revolutionary simulation-guided study design tool that ensures clinical trials are designed effectively to collect the right data, in the right patients, in the right way. Its use supports evidence-based design decisions to extensively de-risk real clinical studies, reducing development time, costs and patient burden.
The Challenge
A privately held biotechnology company at an early stage of clinical development was interested in designing a study using biomarkers to indicate treatment efficacy in subjects with stage 2/3 fibrosis with non–alcoholic steatohepatitis (NASH). NASH is a liver condition characterised by inflammation and fat accumulation and is usually accompanied by fibrosis (Figure 1). Five biomarkers are believed to be involved in the disease process – ELF, PRO-C3, GAL-3, aPAI and YKL-40. The Sponsor wanted an early assessment of the potential efficacy of their treatment within a Phase I/IIa safety and pharmacokinetic (PK) study. As part of this process, they wished to consider the following questions:
- Which of these biomarkers will give the best chance of success in clinical trials?
- Are some biomarkers more variable than others and what impact will that have?
- What if the chosen biomarker/s are more variable than anticipated?
- What is the advantage of using a change from baseline approach in the analysis?
- What sample size should we plan for?
- What effect will different treatment allocation ratios have?
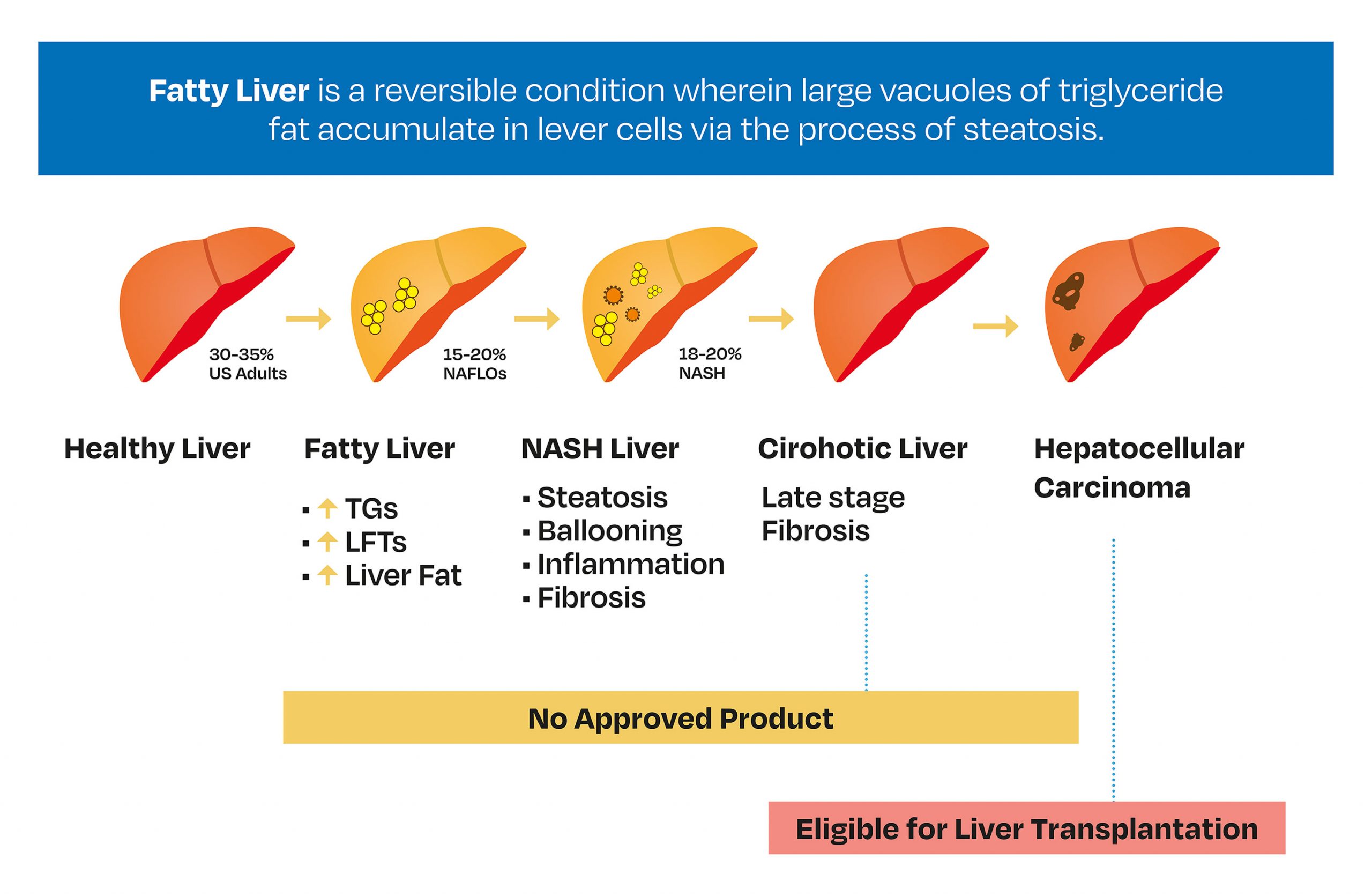
Figure 1. Liver disease progression, where fibrosis is categorised by severity of liver scarring
The Approach
To support the assessment:
- Extensive literature searches were carried out by the Data Science team to identify and collate information on the five biomarkers e.g., expected response in the untreated target population and expected level of variability.
- The data sourced was standardised and synthesised to generate virtual patient level synthetic datasets within KerusCloud to inform study simulations of numerous ‘what if’ scenarios of interest (Figure 2).
- Different study scenarios were used to explore the six key questions of interest for the Sponsor and anticipate issues such as variability that was larger than expected or treatment effect that was smaller than expected. Scenario outcomes were viewed via interactive KerusCloud heatmaps (Figure 3) where they could be explored in detail.
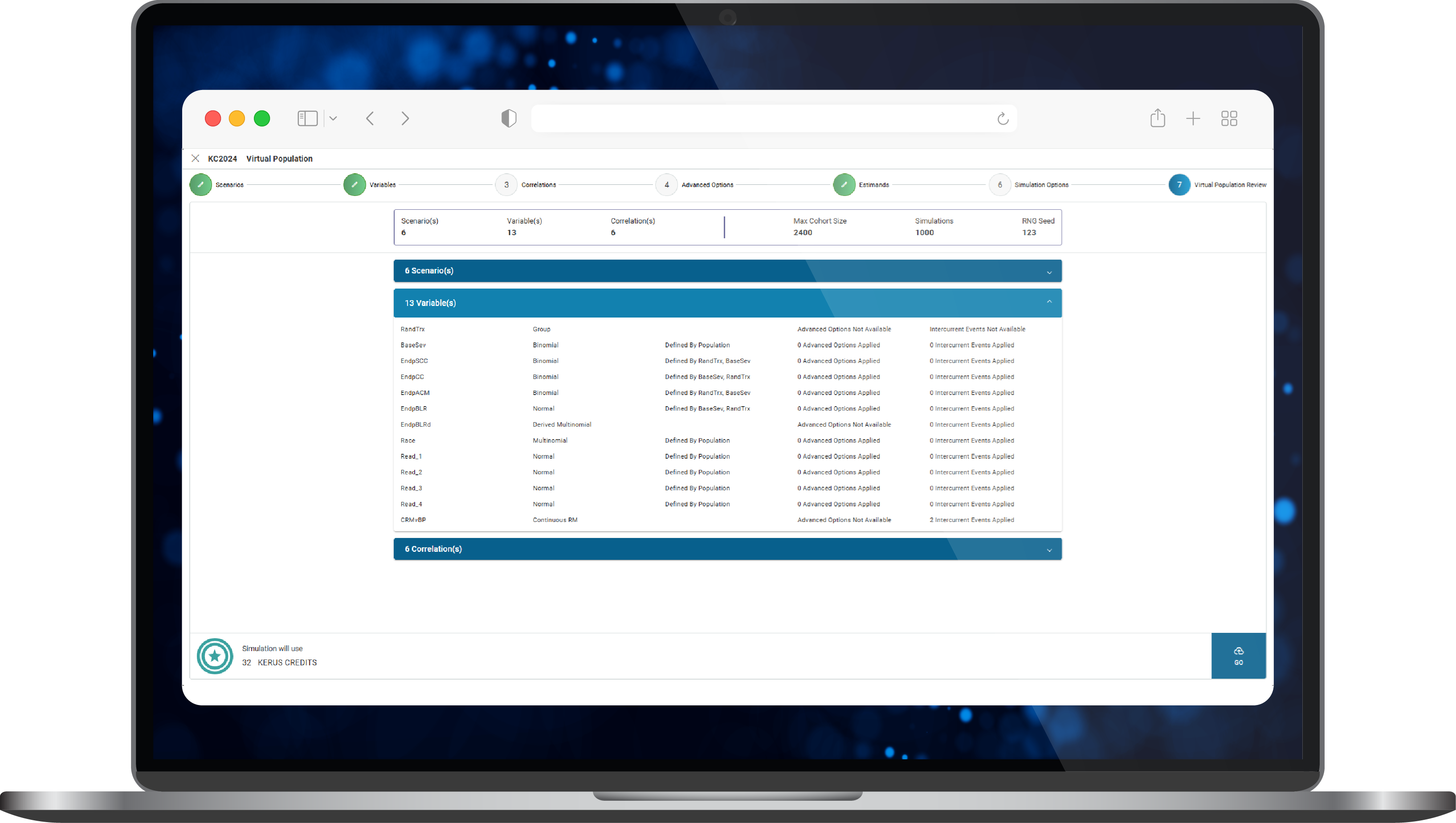
Figure 2. Construction of a virtual population in KerusCloud
The Results
Exploration of multiple scenarios indicated that:
-
- Analysis of covariance including baseline was the most powerful analysis.
- Using PRO-C3 biomarker would lead to the greatest probability of success; with around 35 subjects required to observe a reduction of 50% with 80% probability of success.
- YKL-40 required more subjects to achieve the same level of success as PRO-C3; with over 60 subjects required to observe a reduction of 50% with 80% probability of success.
- A substantial increase in the number of subjects was required if a smaller clinical effect was evident; an increase from 35 to 85 subjects was required if a 33% reduction was observed.
- When moving from a treatment allocation ratio 1:1 to 1:2 to 1:3, the probability of success decreased. This varied according to sample size and biomarker.
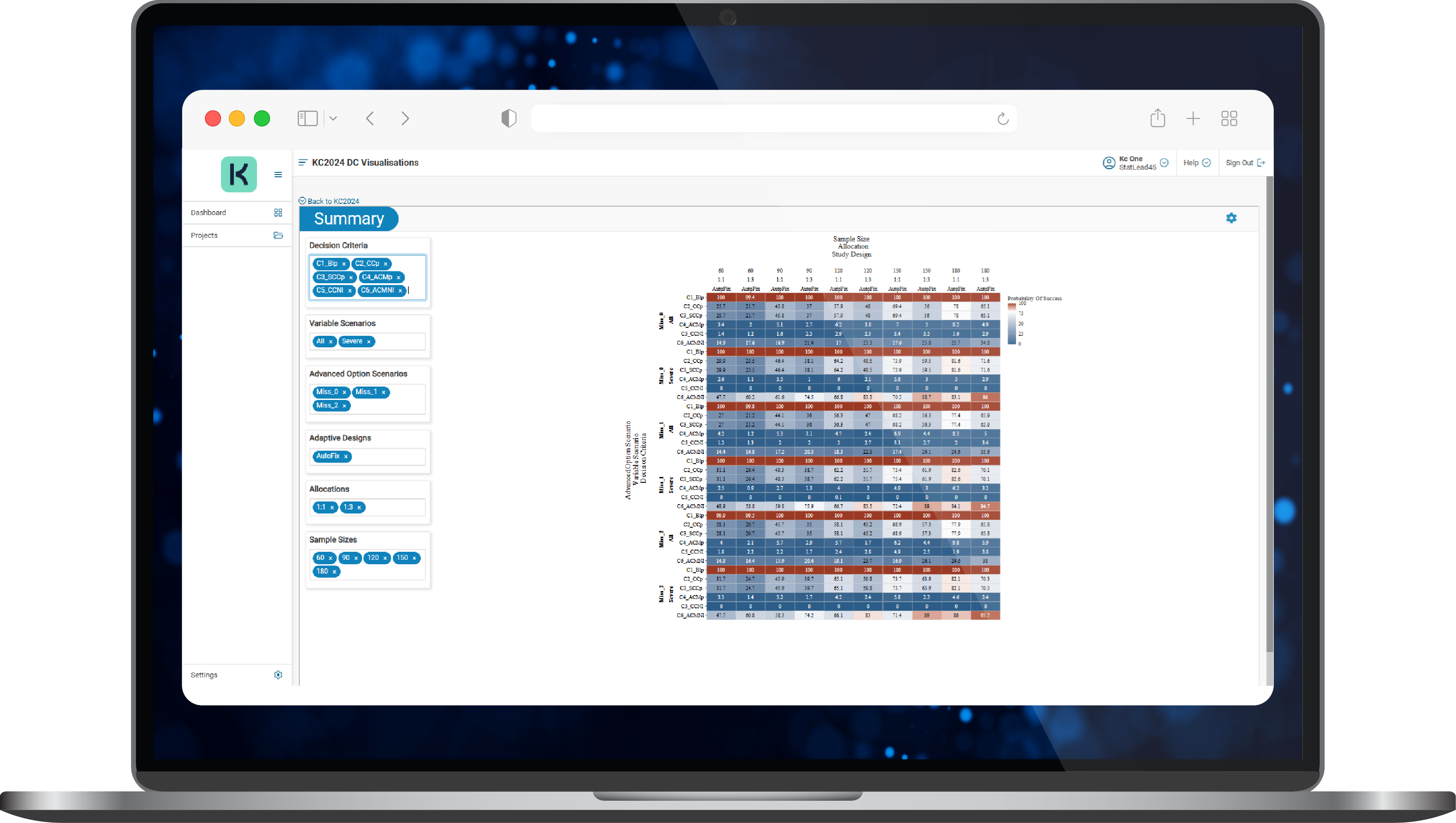
Figure 3. A typical results heatmap in KerusCloud
The Impact
KerusCloud enabled the Sponsor to use quantitative based evidence to make decisions regarding the design of the study. Using KerusCloud established that:
- The inclusion of a panel of biomarkers as secondary endpoints in an early-phase trial with safety and PK as primary endpoints provided a realistic chance of observing a clinically relevant treatment effect.
- Using the biomarker PRO-C3 would give the best chance of success in clinical trials.
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




