Case Study
Identifying the best overall strategy for study designs and drug development pathways
KerusCloud is a revolutionary simulation-guided study design tool that ensures clinical trials are designed effectively to collect the right data, in the right patients, in the right way. Its use supports evidence-based design decisions to extensively de-risk real clinical studies, reducing development time, costs and patient burden.
The Challenge
EU and US regulators are seeking to enable early access to novel treatments for patients with severe unmet need. In the EU, the Medicines Adaptive Pathways for Patients (MAPPs) shown in Figure 1 is an initiative looking at ways to enable early access including:
- Flexible development and access pathways within the current regulatory framework that balance early patient access, public health and societal benefits.
- Early authorization of a product focused on a well-defined and targeted population with a clear safety and efficacy profile.
In collaboration with NICE, MIT and NEWDIGS, Exploristics examined how the MAPPS concept could be applied in development, evaluating the benefits and risks of a treatment, and the commercial impact of the development options on the treatment.
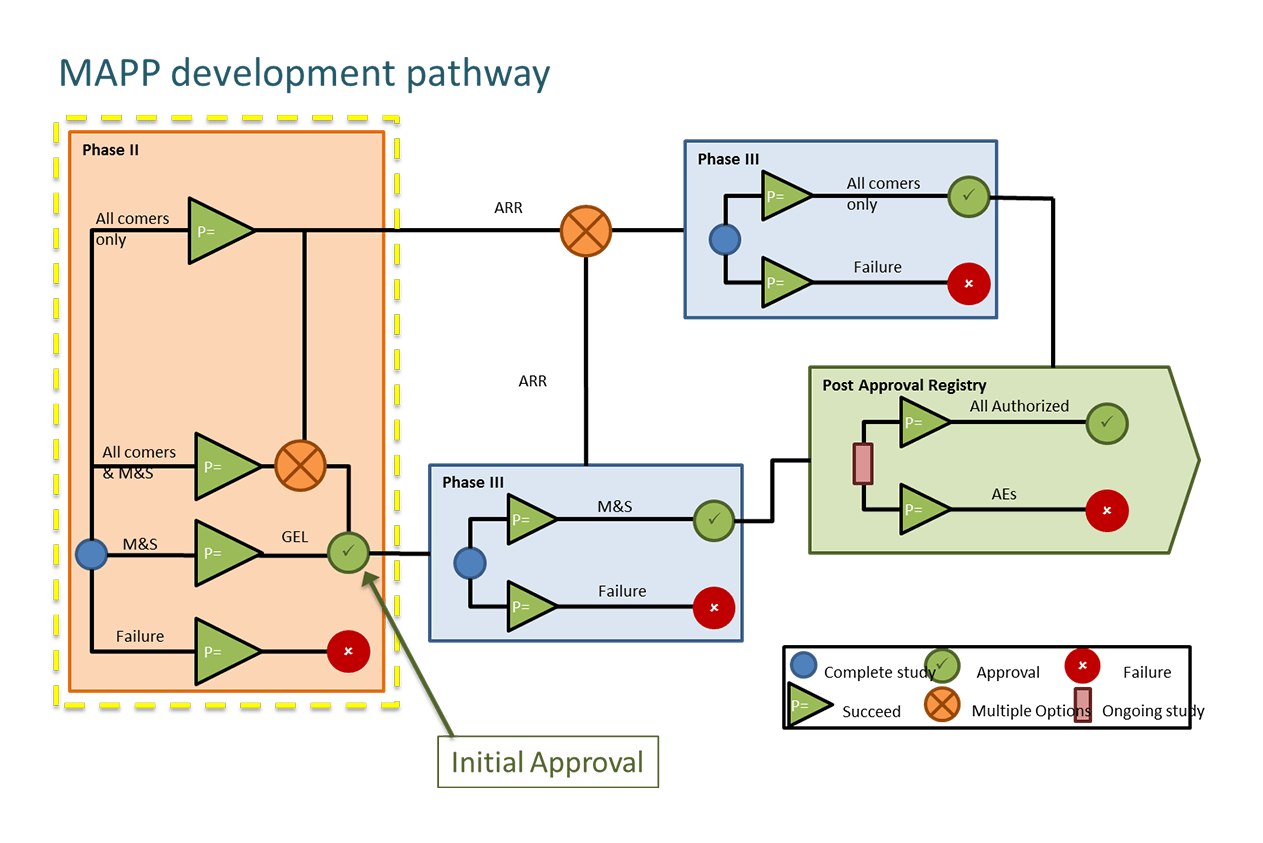
Figure 1. The MAPPs Development pathway.
The Approach
Information on a marketed treatment for relapsing Multiple Sclerosis (MS) was used by KerusCloud to generate realistic patient level data in silico and design alternative MAPP development plan scenarios in a virtual environment to:
- Identify a higher benefit moderate & severe (M&S) sub-population for early authorization and real-world evidence collection.
- Continue to develop the All-comers indication with no launch delay.
- The outputs from KerusCloud including probability of success and sample size were used as inputs for commercial forecasting.
The Results
- The outcomes of simulated scenarios in KerusCloud broadly supported the MAPPs approach and demonstrated that:
- The MAPPs design would provide sufficient evidence for approval in a larger Phase II trial.
- The Phase II trial would need to randomize 450 patients rather than 240 in the original design.
- Initial approval could be in 5 rather than 8 years.
- Financial modelling of the impact of MAPPs for Patients, Payers and Sponsors (Figure 2) indicated an increased expected Net Present Value (eNPV) of $600M rather than $460M for sponsor.
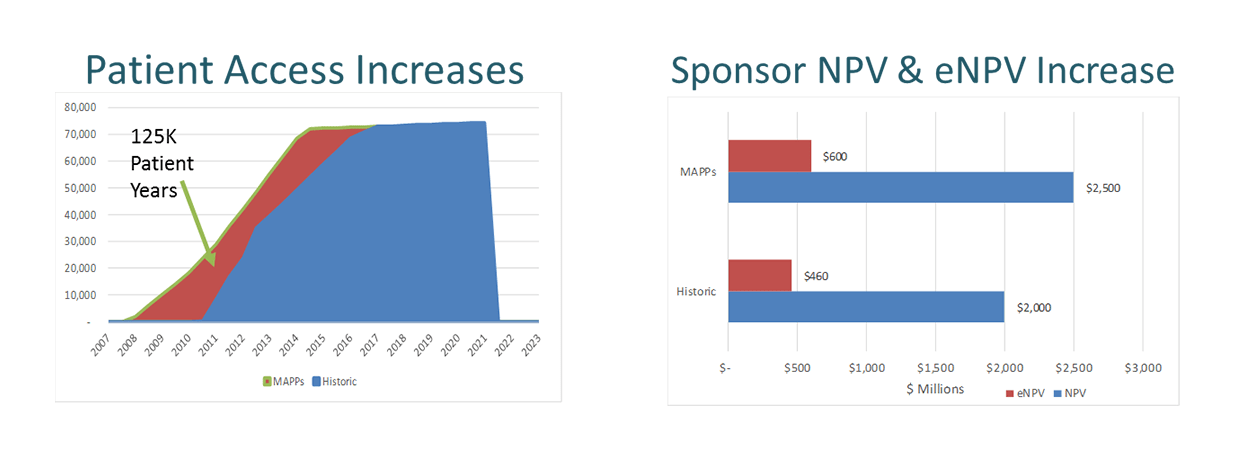
Figure 2. Financial modelling of the impact of MAPPs for Patients, Payers and Sponsors
The Impact
With KerusCloud it was possible to show that:
- The MAPPs approach can provide access for patients up to 3 years earlier.
- MAPPs was beneficial for developer and payer economics.
“Adaptive Pathways integration of staged approvals with real world evidence poses many issues regarding patient safety, patient access, developer incentives and payer affordability. The Exploristics team worked seamlessly with a joint MIT and IMI ADAPT-SMART team to create a case study that quantified not just a single study but an entire adaptive clinical development plan of multiple trials for dual approvals. The work was done in only a few months, demonstrating improved patient safety and increased population benefit inducing further support for adaptive pathways policies.” ”
Mark Trusheim, NEWDIGS Strategic Director, MIT Center for Biomedical Innovation, USA
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




