Don’t roll the dice on
your clinical trial.

Don’t roll the dice on
your clinical trial.

Clinical trials are the cornerstone of medical innovation, helping bring
life-changing and life-saving treatments to market. And yet, so many development
teams roll the dice, sinking time and resources into projects doomed to fail.
Clinical trials are the cornerstone of medical innovation, helping bring life-changing and life-saving treatments to market. And yet, so many development teams roll the dice, sinking time and resources into projects doomed to fail.

De-risking studies, by design
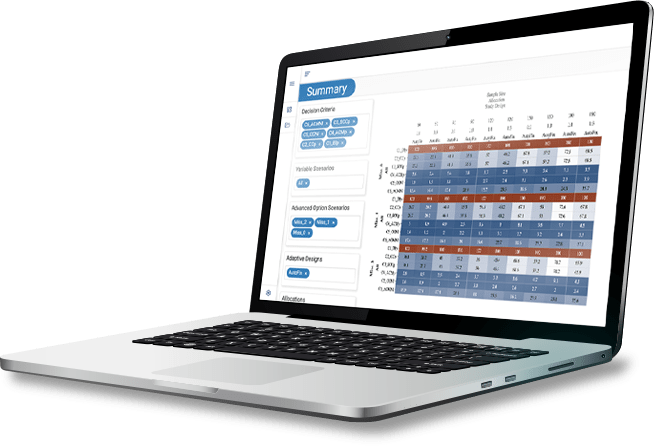
KerusCloud is a cloud-based study simulation platform that allows you to explore multiple study design and analysis options in a virtual environment. It gives you the right data to optimise your design from the outset – and that makes an impact that your team can measure.
Increase in the probability of success for a development program
Decrease in development time for a clinical study
USD saved on a single clinical study
Design for success with KerusCloud

Optimise patient recruitment
- Visualise patient recruitment scenarios. Simulate recruitment rates and site numbers to understand how population choice impacts your study’s length and probability of success.
- Forecast study timelines more accurately. Analyse your recruitment strategies in silico to better understand the operational landscape and impact of potential adjustments to recruitment strategies, sample sizes, and decision criteria, ensuring your real trial stays on track.
Optimise study designs with advanced simulations
- Stay ahead of regulatory requirements. Ensure study designs comply with ICH E9(R1) guidelines. Incorporate estimand strategies that align with FDA and EMA requirements, keeping your study compliant while delivering results ready for regulatory approval.
- Identify the most effective study design: Explore a range of fixed and adaptive study designs in silico to evaluate how they impact study outcomes and enable you to reduce costs, accelerate timelines, and minimise patient burden.
- Enhance trial flexibility and manage uncertainty: Use KerusCloud’s probabilistic framework to estimate the likelihood of different outcomes as new data emerges, and refine your development plan accordingly.


Mimic real-world conditions with synthetic data
- Leverage virtual population data. Manage complex virtual datasets and simulate your study in a way that accurately mimics actual clinical trial dynamics.
- Access predefined data libraries: Rapidly set up simulations that include virtual patient populations, recruitment scenarios and various adaptive design options.
- Streamline trial execution: Save time and resources by creating external control arms, reducing the need for extensive recruitment in clinical trials.
- Maintain patient privacy: Enable the sharing of regulated or sensitive data while maintaining privacy and compliance through anonymisation.
- Address imbalanced or missing data: Generate large, auto-labelled datasets to support predictive modelling, machine learning, and AI so you can address imbalanced data in clinical studies.
Visualise, export, and explore results
- Choose the right study design the first time. Use the interactive heatmap to visualise how different decisions affect the study’s probability of success.
- Support transparent decision-making. Share synthetic data, recruitment profiles, simulation results, and reports with your team throughout your trial.
- Analyse patient populations, recruitment, and trial dynamics. Integrate with R and other analytics tools to create tailored visualisations.

What our customers say

Loic Lhuillier
COO
We were pleased to partner with the Exploristics team on our trial design and statistical analysis, which has greatly contributed to the efficiency of the programme. KerusCloud allowed us to develop our study design to recognise the transformative potential of EXN407 whilst ensuring we account for and minimise any potential risks.

Nicole Cizauskas
Researcher in the Biostatistics Research Group
KerusCloud allows the generation of synthetic data to be expedited while maintaining high data quality. The rapid generation of synthetic data permits these methods to be built better, faster, and include more diverse targets of interest. The support from the Exploristics team has given a unique industry perspective to my research, allowing me to focus on wider accessibility of the final product.

Cristina Olivia
CMO
Gaining the best understanding of data from clinical trials is key to the development of any new drug.
We have been impressed with the experience and support that Exploristics provided us on our oncology and COVID-19 studies, with meaningful insights generated to inform decisions along the development path.
Get in touch to find out more or book a demo.
Want to find out more? Get in touch.