Case Study
Simulating patient level outcome data to inform clinical trial design based on real-world, clinical trial and literature data
KerusCloud is a revolutionary simulation-guided study design tool that ensures clinical trials are designed effectively to collect the right data, in the right patients, in the right way. Its use supports evidence-based design decisions to extensively de-risk real clinical studies, reducing development time, costs and patient burden.
The Challenge
GSK were designing a phase III programme for a novel treatment for anaemia associated with chronic kidney disease in dialysis patients. Both the novel treatment and recombinant human erythropoietin (current standard of care) modulate the Haemoglobin (Hb) levels. However, there were some complexities to consider when designing the study:
- Low and/or very high levels of Hb are both associated with an increased risk of major adverse cardiac events (MACE) which was the primary outcome.
- Baseline risk factors (older age, diabetes, prior myocardial infarction (MI), heart failure (HF), stroke) are also known to increase risk of MACE.
- The complex relationship of varying risks over time in different risk groups at baseline may lead to uncertainty for accurate outcome prediction.
The Approach
KerusCloud was used to simulate realistic patient level outcome data to inform clinical trial design under different scenarios.
- Input data was derived from multiple sources including real-world data, clinical trial data and the published literature.
- A range of study design and analysis options were evaluated for their ability to detect differences in treatment for time to MACE. Design options included studies that were enriched for high-risk patients.
- Additional analysis options included standard cox regression models, adjusted models and time variant models.
- Various metrics were derived from simulated studies including probability of success, study duration and sample size.
The Results
Simulations using KerusCloud identified that:
- Studies enriched with high-risk patients resulted in smaller sample size or duration.
- The analysis approach had minimal impact on study power when Hb levels were well controlled.
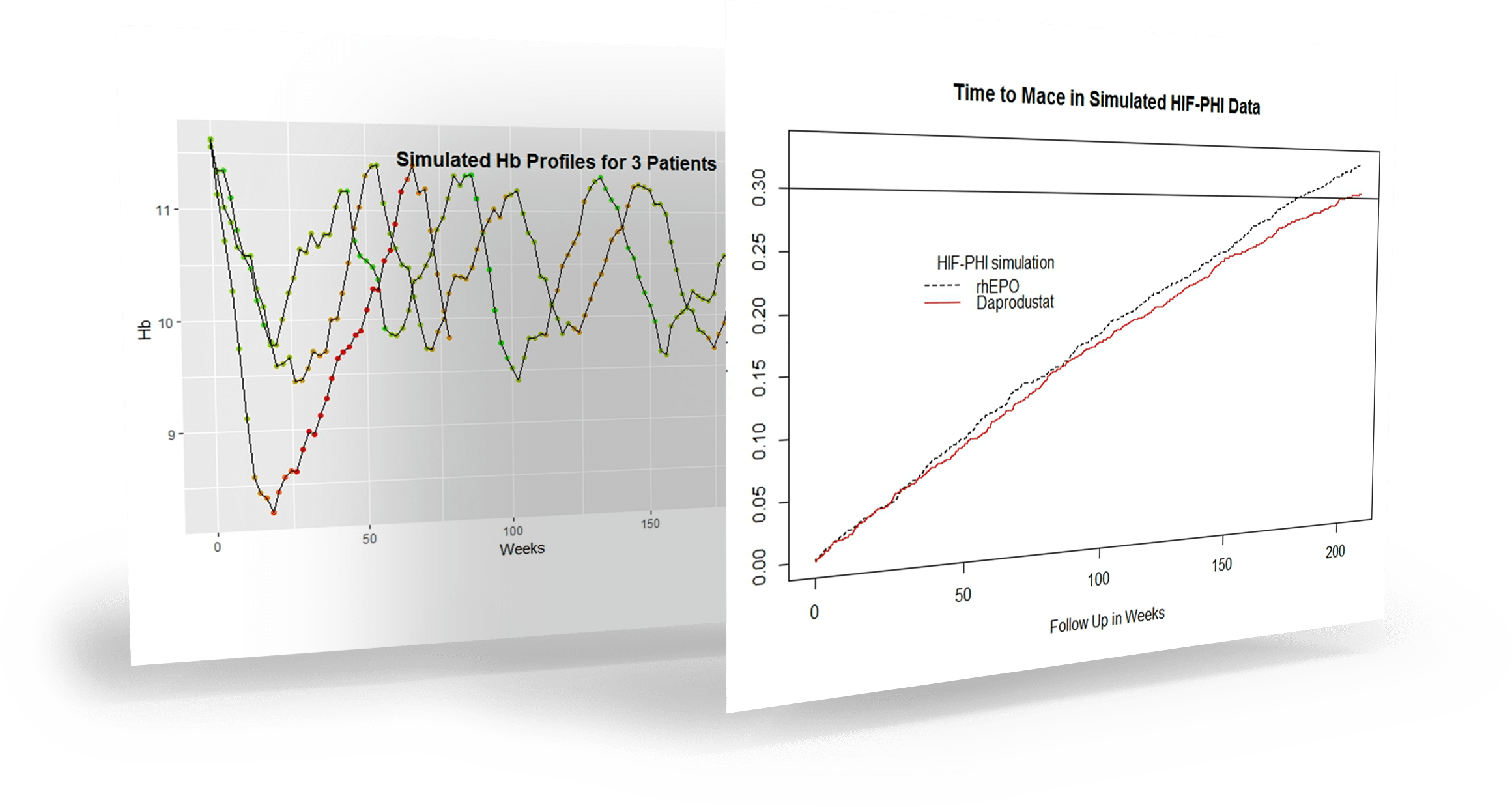
Figure 1. Typical simulated patient profiles
The Impact
- KerusCloud successfully utilised real-world data to inform simulations that helped optimize design and analysis options for a study where there were multiple sources of uncertainty.
- The simulations confirmed the validity of assumptions for the study under different conditions for the design and analysis options and treatment effects.
- Viable alternative study approaches were identified e.g., enriched study design which reduced study duration and sample size by as much as 30%.
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




