Strategic thinking in Biotech R&D: How statisticians can get you investor ready
By Aiden Flynn, Exploristics CEO
At the height of the COVID-19 pandemic, life sciences investment thrived. In the uncertain economic environment of a global healthcare emergency, Pharma and Biotech attracted record levels of investment from life sciences venture capital (VC) and generalist investors alike. Biotech stock valuations and IPO (initial public offering) funding peaked in 2021. However, since then investment has drastically declined, as illustrated by the over four-fold drop in total biotech IPO funding from US$16B (Q1-3) in 2021 to US$3.4B (Q1-3) in 2023 (Figure 1). Such financially straitened circumstances have forced a tightening of R&D belts, with both project pipelines and teams streamlined to retain some financial headroom for key programmes.
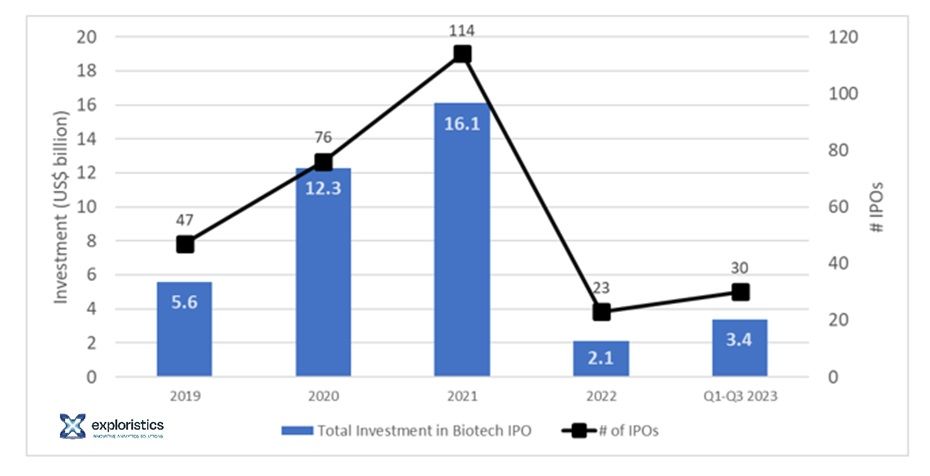
Figure 1. Biotech IPO funding between 2019 and 2023, from McKinsey Insights
Thinking differently
Although life science venture capital has dropped since the dizzy heights of 2021, it has remained above pre-pandemic levels. This is in part due to continued interest in platform led innovations such as machine learning approaches to drug discovery which offer future therapeutics across a wide range of indications. Yet, while the green shoots of a greater recovery in sector investment are waiting to break through, there has never been a better time for Biotech to rethink its approach to derisking R&D.
Risky business
Biotech has always been financially precarious and fraught with risk. This has been down to several common challenges including:
- Uncertain funding due to difficulties in investors valuing pipeline assets making biotech stocks volatile.
- High development costs due to the increasing complexity of the R&D landscape
- R&D productivity limited by relatively small development pipelines often comprised of one/low numbers of investigative assets.
- Regulatory hurdles as the rate at which new technologies and methodologies are developed often exceed regulatory adaptation.
However, the most significant risk to any Biotech is the failure of a lead asset in clinical development. It is well recognised that around 90% of investigational medicines fail during the development process, but this masks some important underlying trends that have a significant impact on Biotech companies. Typically, Biotech looks to find a partner following proof of concept and mid-stage/phase II trials, but these are the riskiest of all with a failure rate around 70%. Having only a few assets in your development basket is not for the faint-hearted and only emphasises the importance of managing the risks.
A silver lining
Tighter R&D budgets are uncomfortable but can provide a silver lining to the financial cloud by encouraging more strategic thinking around how best to use them. R&D has become increasingly expensive while high attrition rates have failed to budge. Leaner times offer a valuable opportunity to evaluate project risks more closely earlier and so target limited resources more effectively. Here a good statistician can be your best asset.
Statistical solutions
Engaging with a statistician early can really help you make the most of the development budget you do have. They can:
- Help you scope the project, staging it so that spend is prioritized according to study impact, quantifying expected spend alongside likelihood of success at each stage. This ensures you have the data needed to decide where best to focus available budget so you can build the most robust evidence package possible for the next funding round or out-licencing.
- Differentiate your proposition to investors with data-driven derisked development plans, soothing fears and helping you stand out from the crowd.
- Evaluate the clinical, statistical and operational risks to identify which studies are most likely to fail. This ensures budget and resource is not wasted on unnecessary studies.
- Prioritise indications for your asset ensuring you pick the best one first, so you don’t fall at the first hurdle.
- Identify the most efficient design for your study ensuring you have the right population, the right endpoint and the right analysis strategy and decision criteria. The right design also overcomes potential recruitment issues, minimises patient burden and improves compliance.
Winning over investors
Despite recent rocky investment, the Biotech sector remains resilient and is a powerhouse for life science innovation. Biotech companies are increasingly recognised as valuable early-stage research engines, bringing high quality new drugs into the drug development process. However, to weather a tougher economic climate and attract financing it helps to cut out waste, reduce risk and present the most compelling investment case you can. As you navigate your Biotech through current choppy waters, chart your course to better financing by making sure that a statistician is your very first port of call.
Read more:
Burgeoning Biotechs: What are the pros and cons of hiring your own statistician
Open Road: Being a statistician in a small biotech
Designing clinical studies for success
Revolutionising clinical trial design with in silico studies
Statistical Consulting Services
Watch more:
Common causes of clinical trial failure
Can pharma learn from development in other industries?
Matching trial objectives to design using the estimands framework




