Case Study
Building and validating a composite biomarker model to predict emphysema progression
KerusCloud is a revolutionary simulation-guided study design tool that ensures clinical trials are designed effectively to collect the right data, in the right patients, in the right way. Its use supports evidence-based design decisions to extensively de-risk real clinical studies, reducing development time, costs and patient burden.
The Challenge
Global pharmaceutical company GSK was seeking to build a predictive model to identify patients with worsening emphysema for selecting the right population in chronic obstructive pulmonary disease (COPD) studies. They wanted to understand if KerusCloud could be used to design the study given that:
- There is no standard approach for designing a validation study for a multivariate model.
- The ability to build and validate a model was limited by the number of available samples.
The Approach
Working in collaboration with GSK COPD Clinical Discovery, Exploristics used the GSK ECLIPSE study as a basis for building a predictive model involving multiple baseline biomarkers.
- The biomarker and clinical characteristics as well as the correlation structure was extracted from patient level ECLIPSE data.
- Validation comprised multiple study objectives relating to the performance of individual markers as well as the overall model.
- A range of study designs was evaluated for their ability to achieve multiple objectives and a full model (8 biomarkers) and a reduced model (5 biomarkers) were assessed with results explored via a heatmap (Figure 1).
- Various metrics were derived from thousands of simulated studies including an assessment of the model performance and p-values for individual biomarkers.
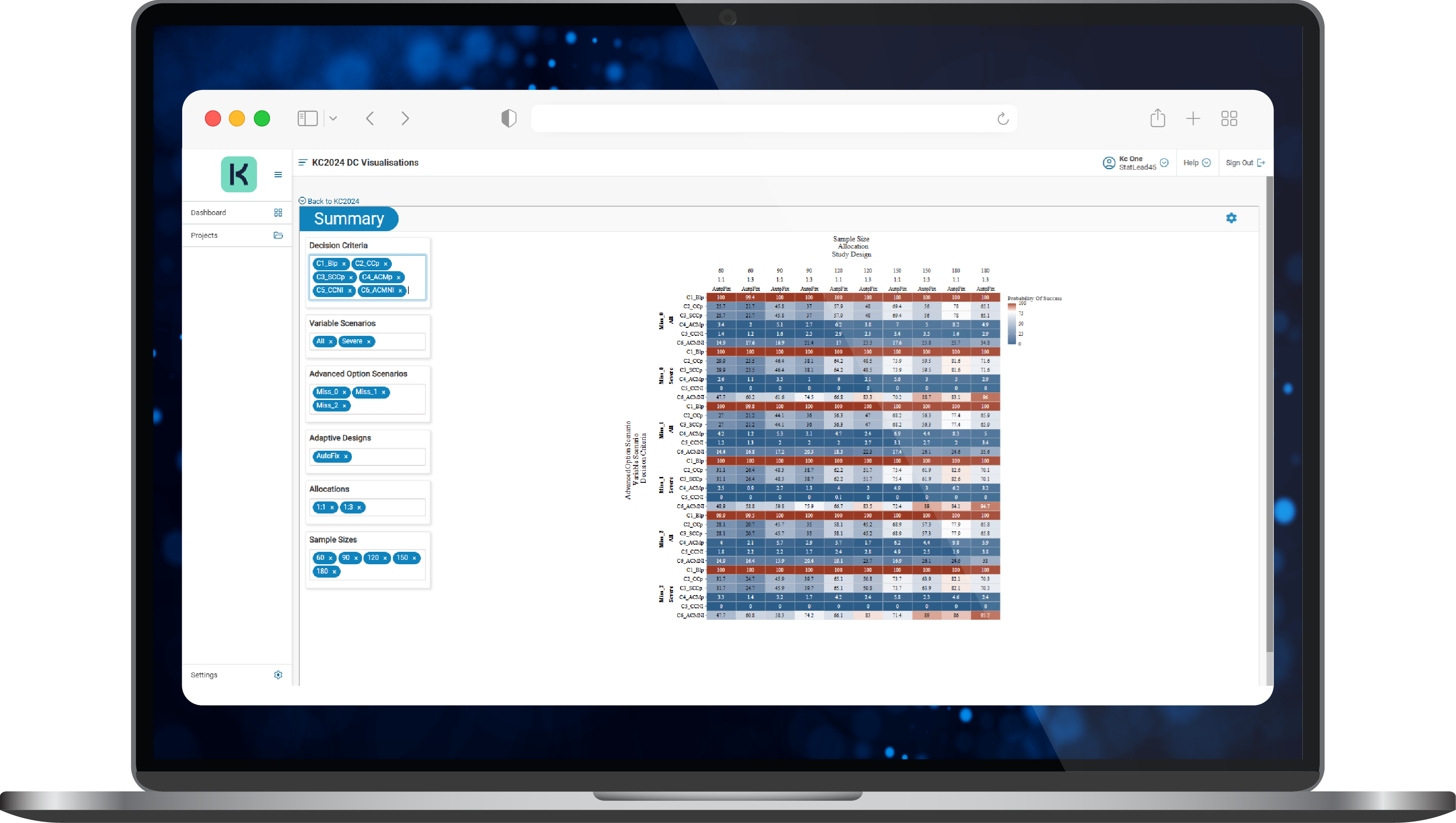
Figure 1. A typical KerusCloud results heatmap
The Results
- KerusCloud generated realistic data which could be used to evaluate the overall performance of the model and individual markers (Figure 2).
- The full model required > 200 samples whilst the reduced model required only 60 samples.
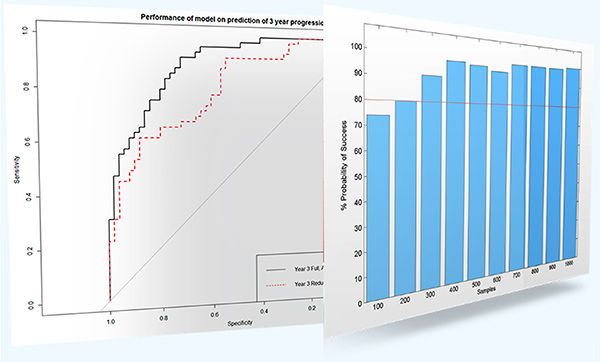
Figure 2. Assessment of model performance and optimal sample size.
The Impact
The study demonstrated that KerusCloud could:
- Generate realistic data in silico and be used to design validation studies for multivariate predictive models.
- Optimize the model building and validation strategy given the available samples.
- Deliver a strategy that required 70% fewer samples for the same probability of success.
- Reduce the risk of attempting a study with a low chance of success.
“We were extremely pleased with the results of the work. Until Exploristics became involved we were struggling to come to conclusions about the utility of the biomarkers being evaluated. The results with KerusCloud provided our team with a solid understanding of the data allowing clear conclusions to be drawn.”
Senior Scientific Director, Respiratory TAU, GSK
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




