Case Study
Identifying disease areas and endpoints that best demonstrate treatment efficacy
The Data Science team provide advanced analytics using proprietary algorithms, tools, machine learning and predictive modelling approaches to extract insights and synthesize evidence that can be used to inform the assumptions, strategy, and direction of any clinical trial program.
The Challenge
A biotechnology company was developing an investigational drug which was not disease specific.
The drug targeted a specific molecular pathology and operated through a mechanism of action within a particular signalling pathway. To support its development, the company wanted to identify:
- Potential disease domains for the investigative agent
- Endpoints for demonstrating treatment efficacy
- Biomarkers of interest
The Approach
Identifying potential disease domains
Using proprietary tools and Clinical Trial database access, the Data Science team:
- carried out a literature review to understand key biological aspects of the treatment.
- conducted eight custom searches targeting key mechanisms of action within specified cellular pathways, reviewing information from 2,630 trials to identify disease domains whose interventions shared similar mechanisms of action.
- extracted data covering 25 interventions across 1,074 trials for those domains.
Identifying information on endpoints and biomarkers using the Pulmonary Suite
Exploristics’ Pulmonary Suite (Figure 1) is a collection of data models containing information from 23,402 clinical trials, the published literature, real-world data and disease registries.
As several pulmonary indications were identified as potentially likely to show efficacy for the investigative treatment in the initial disease domain searches, the Data Science team searched the model suite for further information for those indications that could help:
- Identify critical endpoints for demonstrating treatment efficacy.
- Determine, through a meta-analytical approach, suitable assumptions to use as simulation parameters for the identified critical endpoints. This would later aid in the determination of a suitable and pragmatic study design.
- Identify and determine endpoint relationships to better construct realistic virtual patient data for study design purposes. This allowed for the comparison of multiple correlated endpoints during a later simulation exercise to inform the study design.
- Identify and evaluate key biomarkers of interest and their documented relationship to regulatory approved endpoints or approved surrogate endpoints.
Figure 1. Development stage overview of the Pulmonary Suite, showing stage of completeness by number of trials in each disease area. The Pulmonary Suite offers a selection of expertly curated and synthesised historical data across multiple pulmonary diseases. It includes disease models at four complexity levels: 1) landscape overview, 2) standardised endpoint, 3) standardised data and 4) comprehensive data model. Indications in the suite include pulmonary artery hypertension (PAH), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), asthma, COVID-19 (CV-19), bronchiectasis (B), influenza (Flu), non-small cell lung cancer (NSCLC), rhinovirus (hRV) and respiratory syncytial virus (RSV).
Figure 1. Development stage overview of the Pulmonary Suite, showing stage of completeness by number of trials in each disease area. The Pulmonary Suite offers a selection of expertly curated and synthesised historical data across multiple pulmonary diseases. It includes disease models at four complexity levels: 1) landscape overview, 2) standardised endpoint, 3) standardised data and 4) comprehensive data model. Indications in the suite include pulmonary artery hypertension (PAH), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), asthma, COVID-19 (CV-19), bronchiectasis (B), influenza (Flu), non-small cell lung cancer (NSCLC), rhinovirus (hRV) and respiratory syncytial virus (RSV).
Simulating clinical studies using reliable assumptions to optimise design
Relevant endpoint and biomarker information identified and selected using the Pulmonary suite was then used as the basis for simulating ‘what if’ study scenarios using Exploristics’ simulation guided clinical study design platform KerusCloud to explore varied study outcomes (Figure 2). Simulation of virtual patient level data are heavily dependent on sourcing reliable assumptions. The pulmonary suite provided a utilisable source of collated and structured information to allow for the simulation of:
- Multiple correlated clinical endpoints to assess the best performing endpoint in relation to the study probability of success and associated sample size, as well as determining the implications of including co-primary endpoints. This included the ability to review different multiple testing strategies through KerusCloud.
- Intermediatory timepoints prior to planned final data time, for the evaluation of adaptive design strategies including, futility analysis, group sequential designs and sample size re-estimation.
- Targeted biomarkers and their relationship to clinical outcomes to aid in the determination of go no-go strategies for study success, primarily based on early biomarker impact and a positive direction on clinical outcomes, supporting both current and future phase
study designs.
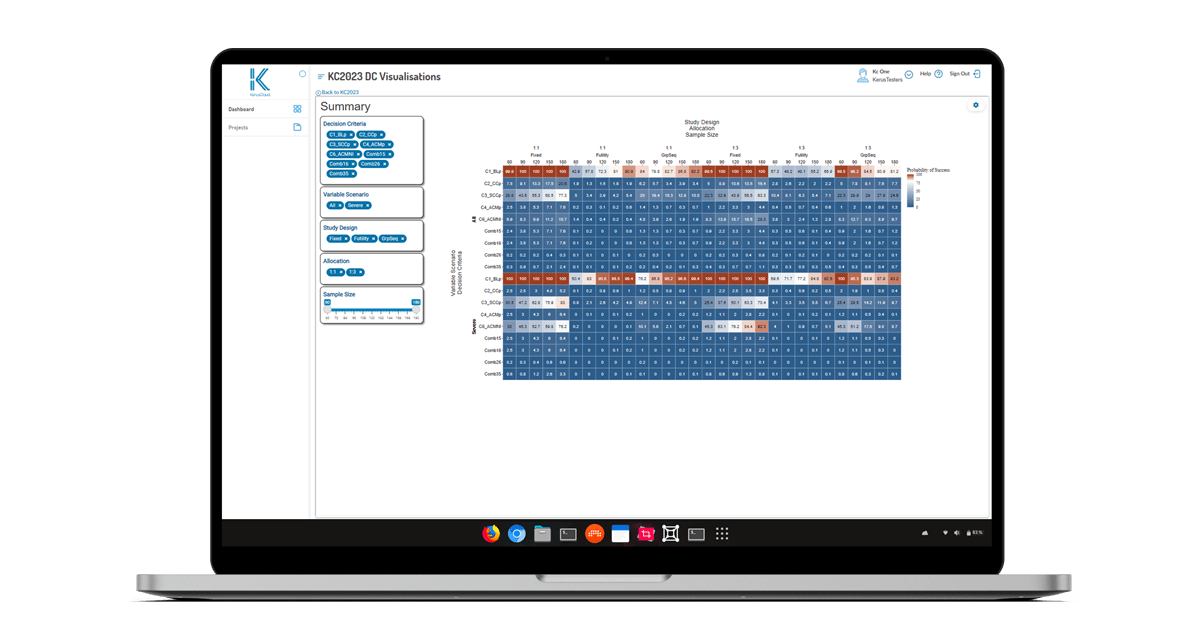
Figure 2. A typical results heatmap in KerusCloud indicating simulated scenario outcomes quantified in terms of probability of success (POS), where blue indicates low PoS and red indicates high PoS.
The Results
Work on this project by the Data Science team resulted in:
- 4 pulmonary disease domains being identified for further exploration with the investigative drug from an initial pool of 500 diseases with interventions that shared the same mechanism of action.
- 4 key biomarkers being identified from an initial 103 exploratory biomarkers as potentially informative for the most promising disease areas.
- critical endpoints and endpoint relationships identified using simulated ‘what if’ scenarios.
The Impact
For the Sponsor, the project yielded:
- Fast track and Orphan Drug Status for the investigative treatment was granted by the
FDA in one of the identified disease domains. - Justification for study endpoints, study size and duration.
“The Kerus platform uses real world evidence and historic data to enable exploration of trial endpoints and routes. We are looking forward to seeing the impact of the approach on expediting development of our lead asset and pipeline projects.”
CEO, Small Biotech, UK
Let’s talk!
If you’d like to discuss this case study further or learn more on how our technology enabled services can support your development project, please contact our VP of Sales & Marketing, Abbas Shivji, at abbas.shivji@exploristics.com or book a call.




