By Kim Hacquoil, Exploristics CDSO
Respiratory diseases are among the most widespread, challenging, and life-threatening conditions that humanity faces. More than half a billion people suffer from chronic respiratory diseases worldwide, and only heart disease and cancer account for more deaths in the UK each year, with one in every 14 fatalities related directly to respiratory diseases [1]. Health outcomes for respiratory diseases have not improved in the UK over the last decade while treatment of lung conditions currently costs the National Health Service (NHS) in the UK £11 billion each year.
Building a more proportionate response
Since the COVID-19 pandemic, there has been increased global attention on respiratory health with a focus on understanding the pathophysiology of respiratory infections and developing effective treatments. However, despite this some organisations suggest more respiratory research is urgently needed to provide an R&D response proportionate to the impact of these diseases on health worldwide.
Respiratory research challenges
Respiratory medicine presents a unique set of challenges for clinical researchers and healthcare professionals, and the field is undergoing a profound transformation with advances in technology, the need for flexibility in trial designs and collaborative strategies driven by continued unmet clinical need. These new approaches are offering opportunities to address some of the key challenges encountered in running respiratory clinical trials, including finding, monitoring and evaluating patients.
Finding patients
In common with many other therapeutic areas, recruitment into respiratory trials is struggling. The number of patients accessing industry research has fallen by 44% between 2017 and 2021 [2] and according to a study published in BMJ Open Respiratory Research [3], 26.3% of trials fail to recruit to their target sample size. However, multiple strategies are now being employed to overcome the challenges. For example:
- Integration of clinical trial recruitment into clinical care pathways utilising electronic healthcare data is being used to improve recruitment and streamline the clinical trial process. This has been shown to significantly boost recruitment into the trial and maximize the generation of adequate data for respiratory research.
- Clinical trials are also increasingly adopting a personalized medicine approach, which involves stratifying patients based on specific disease subtypes, genetics, and biomarkers. Utilisation of historical data and information can optimise clinical trial design to ensure successful recruitment of these patients.
Monitoring patients
Monitoring vulnerable patients with chronic respiratory disease has traditionally posed a significant challenge to clinical researchers. However, patient monitoring is now being revolutionised by the incorporation of digital health technologies, such as wearable devices and mobile apps which have become invaluable in respiratory clinical trials and opened new avenues for remote patient monitoring and real-time data collection. The use of health apps has risen steeply in the last few years, with recent estimates of around 560 million health users in 2022, up 22.5% from the year before.
While some challenges remain around integrating the information derived from digital health technologies, these tools can enhance the efficiency and accuracy of clinical trials, allowing researchers to gather data on patient behaviours, symptoms, and treatment adherence more comprehensively. More accurate and exhaustive datasets supported by efficient remote monitoring can improve trial design through utilisation of adaptive trials which respond in real-time to emerging information and allow for better informed clinical decisions about patient care, treatment plans and enrolment as well as patient engagement.
Evaluating patients
Evaluating the effect of respiratory diseases in patients is complex, and disease progression has traditionally been difficult to measure accurately. Conventional techniques for assessing lung function can be crude, for example the common trial endpoint of a six-minute walk test is not a direct measure of chronic obstructive pulmonary disease (COPD) progression. However, new ways of measuring patient benefit are now emerging. For example:
- Advances in imaging techniques now enable detailed examination of millimetre-level changes in airway wall thickness and tissue densities for distinct regions of the lung. This represents a more precise and non-invasive approach to assess lung function and monitoring disease progression.
- The rise of digital health technologies like wearables has significantly enhanced the real-time monitoring of lung function and disease progression. Notable examples include Wrist-Wearable Actigraphy, which continuously track patient activity and sleep, revealing the real-world impact of respiratory conditions on daily life and treatment effectiveness. Cough Count Wearables accurately monitor cough frequency, helping to assess the severity and progression of respiratory conditions.
- Another innovation, the BioSticker, is a biosensor that captures medical-grade data including vital respiratory parameters aiding in remote patient monitoring and data collections.
Leveraging data
The rising importance of digital health technologies in monitoring and evaluating patients in personalised approaches to respiratory research and treatments can be seen in the growing trend in the use of technology-driven terms like ‘device’, ‘sensor’ and ‘wearable’ in respiratory clinical trials (Figure 1). While these technologies offer transformative solutions to tracking patient health, their impact on respiratory R&D will be dependent on being able to leverage the information that the data holds. Analysing and assessing historical and emerging data related to these novel endpoints will be key to successfully integrating this information into the future clinical trial design and treatment paradigm.
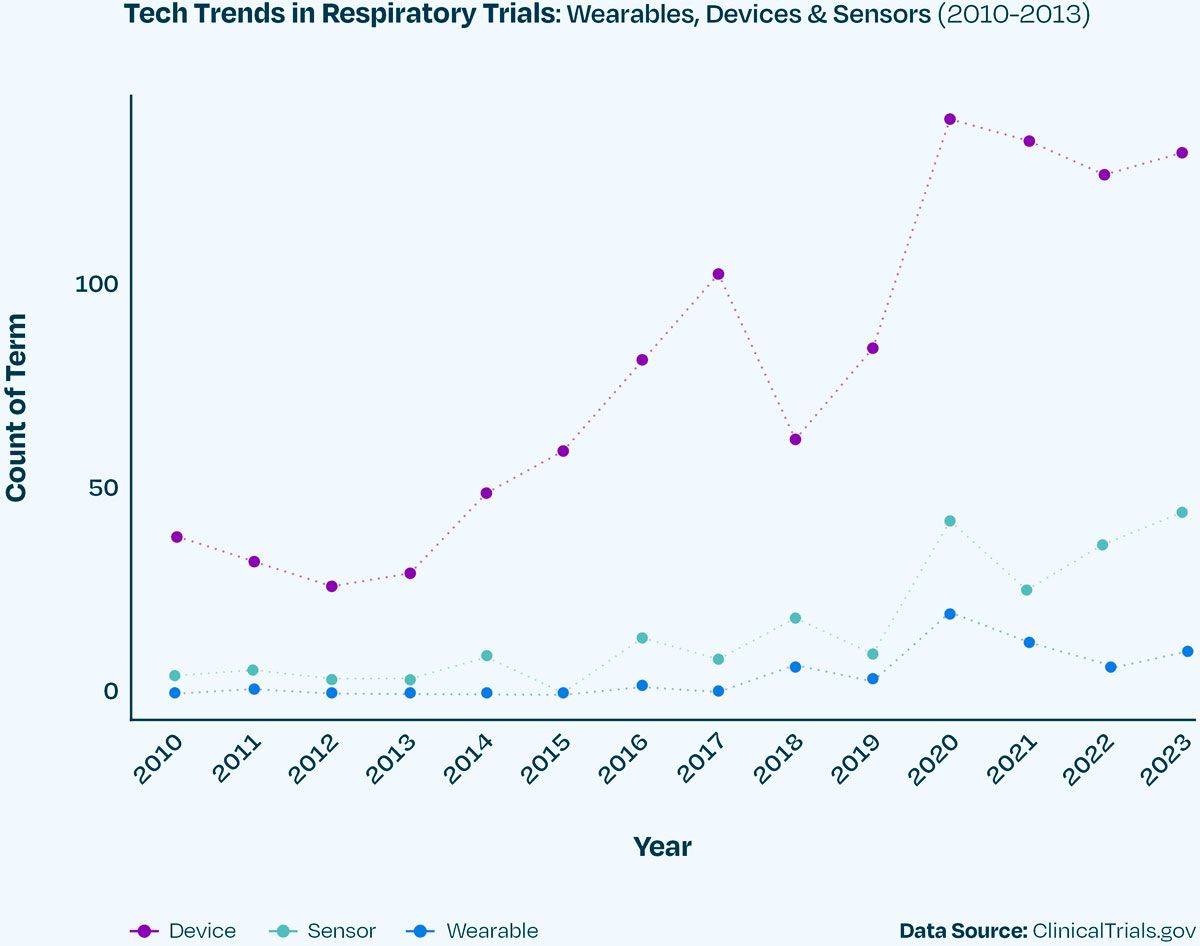
Figure 1. Utilization of terms related to ‘device’, ‘wearable’ and ‘sensor’ within respiratory clinical trial endpoints from 2010 to 2023.
A shifting landscape
In recent years, the landscape of respiratory clinical trials has seen a shift, with the proportion of companies sponsoring a single trial increasing from 1% in 2005 to 10% in 2023 (Figure 2). The opposite is true at the other end of the scale, with the percentage of trials lead by companies overseeing more than 100 trials in total having decreased from 74% to just 25%. This departure from the dominance of larger corporations creates a dynamic market space, offering many opportunities for smaller biotech firms to contribute to and thrive in the world of respiratory clinical trials.
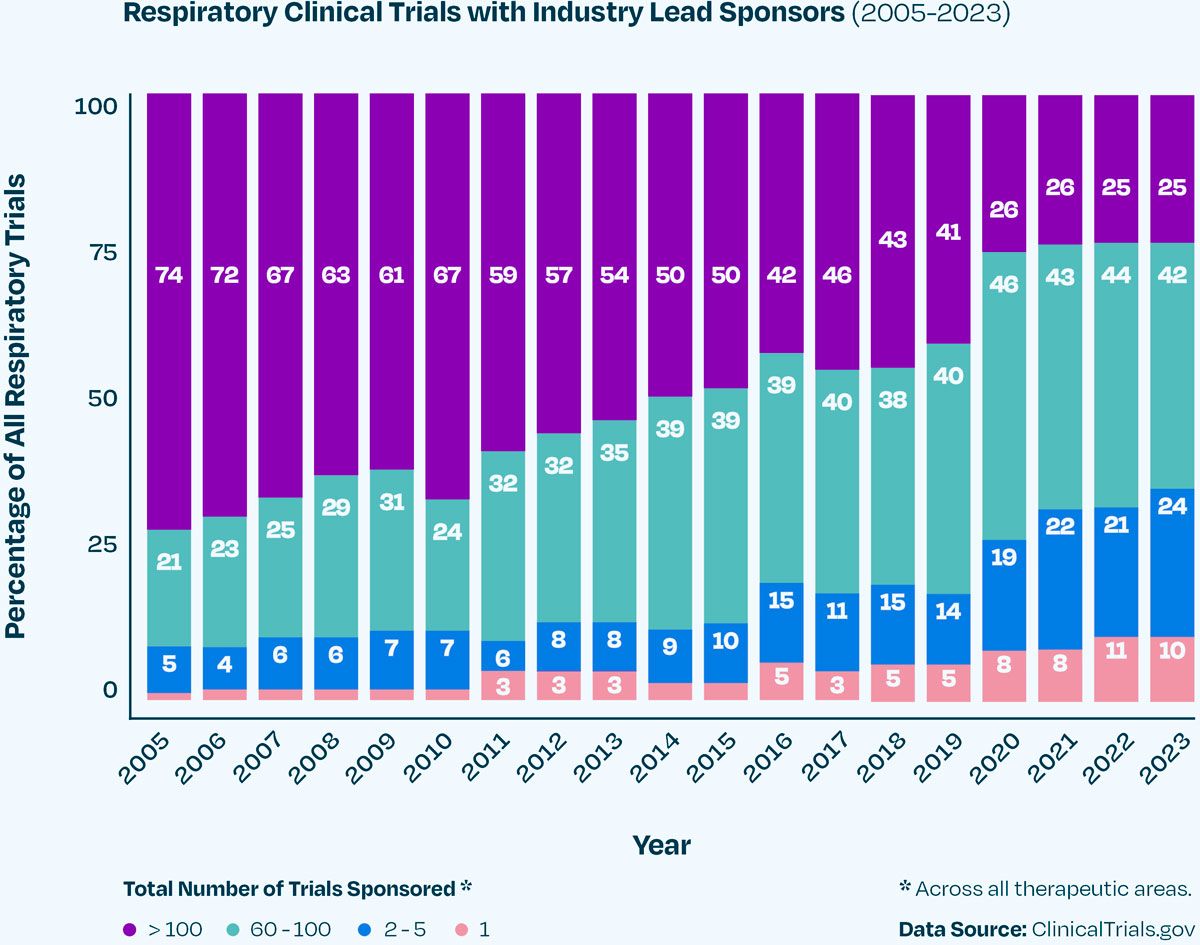
Figure 2. The proportion of respiratory clinical trials lead by an industry sponsor from 2005 to 2023. Companies are categorised based on the total number of trials they sponsor, as a lead or collaborator across all therapeutic areas.
Breathing new life into respiratory clinical trials
Research into respiratory diseases currently accounts for one in every 20 clinical trials happening around the world, with thousands of respiratory clinical trials actively recruiting volunteers. However, while respiratory disease is the third biggest cause of death in countries like the UK, respiratory trials only rank 10th in terms of clinical trial volume.
With the right data partner, emerging digital technologies and data approaches now offer researchers an unprecedented opportunity to better understand the historical and current clinical trial landscape to help guide more targeted respiratory therapies. Given the pace of digital innovation and growing demand for improved interventions, with the respiratory drug delivery market expected to reach $87,745 million by 2029, there has never been a better time to breathe new life into respiratory research.
References
- Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017 – PMC (nih.gov)
- Rescuing patient access to industry clinical trials in the UK (abpi.org.uk)
- Increase in recruitment upon integration of trial into a clinical care pathway: an observational study | BMJ Open Respiratory Research
Read more:
Pulmonary disease specific data models
Using pulmonary disease data models to optimise respiratory clinical trials
Disease specific data model use to inform clinical trial assumptions
Revolutionising clinical trial design with in silico studies
Watch more:




